Jiwon Lee, M.D.
Department of Laboratory Medicine
Background of the Test
Antibodies against human leukocyte antigen (HLA) develop when patients encounter alloantigens from others, typically through pregnancy, blood transfusion, organ transplantation, or human-derived drug administration. For patients requiring organ transplantation, it is crucial to evaluate the HLA antigens of the donor and recipient and check for HLA antibodies present in the patient’s blood. This is particularly important for patients undergoing organ transplantation who might have developed HLA antibodies due to previous transfusions, pregnancies, or prior transplants, potentially leading to sensitization to HLA antigens.
Principles and Types of Testing
Various tests are performed to assess HLA antibodies, including the HLA crossmatch test, panel reactive antibody (PRA) test, Luminex HLA antibody test utilizing Luminex technology, and donor-specific antibody (DSA) test.
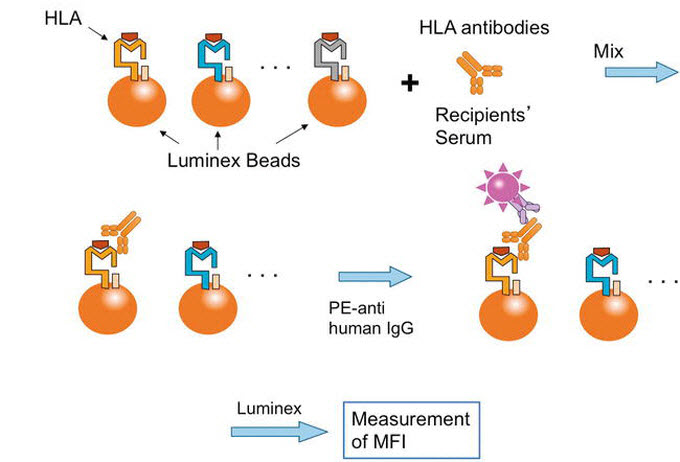
Fig. 1. Schematic presentation of single antigen bead assay (SAB)
Ref) Human Leukocyte Antigen (HLA). Donor-Specific Anti-HLA Antibodies in Organ Transplantation:
Transition from Serum DSA to Intra-Graft DSA (2018)
1) PRA Test
The PRA test is conducted to detect HLA antibodies in the serum of brain-dead organ transplant patients and determine their specificity. The patient’s serum is mixed with a panel comprising lymphocytes from patients with known HLA antigens, and the presence of PRA is assessed. If the result is positive, the reaction rate (%PRA) and specificity of HLA antibodies among the lymphocyte panel are determined. The purpose of PRA testing is to evaluate the degree of sensitization to HLA antigens in the patient, predict the likelihood of finding a compatible donor through crossmatching, and identify unacceptable antigens that should be avoided during donor selection. The traditional PRA test, which uses panels composed of 30–60 lymphocytes, has been replaced by commercial solid-phase immunoassay kits due to challenges in panel construction and the complexity of the testing method.
2) Luminex Bead Assay
The Luminex Bead Assay uses polystyrene beads with fluorescently labeled HLA antigens attached. This test detects HLA antibodies in the patient’s serum by allowing them to react with beads carrying HLA antigens. The fluorescence intensity is measured using Luminex technology, providing information about the presence of HLA antibodies (phycoerythrin [PE] fluorescence) and their specificity (bead fluorescence).
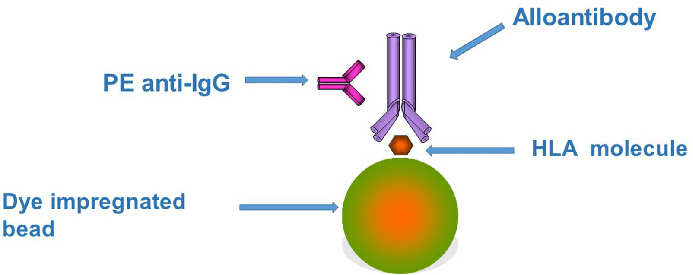
Fig. 2. Principles underlying the Luminex bead assay
Ref) Human Leukocyte Antigen (HLA). Donor-Specific Anti-HLA Antibodies in Organ Transplantation:
Transition from Serum DSA to Intra-Graft DSA (2018)
Based on the abovementioned principles, the Luminex testing method uses polystyrene beads stained with two different fluorescent dyes in varying ratios, theoretically allowing discrimination of over 100 different bead populations. HLA antibodies in the patient’s serum interact with beads carrying the attached HLA antigens. Detection of HLA antibodies is achieved using a fluorescent substance (PE) labeled with anti-human IgG antibodies. Luminex testing demonstrates superior sensitivity and specificity compared to complement-dependent cytotoxicity or enzyme immunoassays. It requires only a small amount of sample and uses a 96-well microplate format, enabling the simultaneous processing of several tests. This methodology presents advantages in terms of efficiency and throughput.
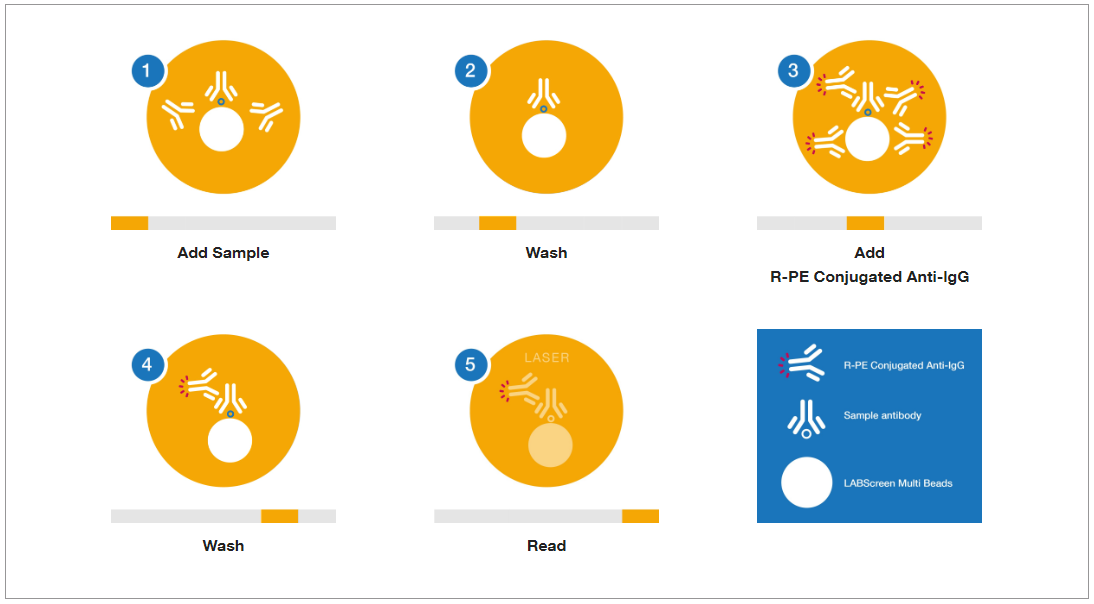
Fig. 3. Principle of HLA antibody testing using Luminex assay
Ref) https://www.thermofisher.com/onelambda/wo/en/pre-transplant/antibody-detection/labscreen.html
The Luminex HLA antibody test can be categorized into three types based on the target antigens attached to the beads:
(1) PRA Screening Test: Detects the presence of HLA antibodies and determines whether the patient has class I or II antibodies using a panel with antigens pooled from multiple donors.
(2) PRA Identification Test: Determines the range (%PRA) and specificity of HLA antibodies in reaction with a panel of antigens from a single donor.
(3) HLA Single Antigen Identification Test: Identifies specific antibodies to individual class I or II recombinant antigens attached to single beads, providing accurate detection of antibodies unidentified in screening or identification tests.
Considerations in Test Interpretation
Luminex testing provides median/mean fluorescence intensity (MFI) values as indicators of antibody concentration; however, caution is advised in interpreting MFI values as they may be influenced by variations in antibody concentration. High concentrations of antibodies can lead to low measurements due to the prozone effect. The results of HLA single antigen identification tests are specific for IgG antibodies and do not assess cellular toxicity. Interferences from high-dose intravenous immunoglobulin, C1 immune complexes, and IgM immunoglobulin may cause false-positive or false-negative results. In such cases, treatments such as heat, dithiothreitol (DTT), or pre-treatment with ethylenediaminetetraacetic acid or DTT can be applied to prevent interference-related errors.
Clinical Significance
HLA antibody detection in patients undergoing organ transplantation is crucial for predicting outcomes, preventing rejection reactions, and guiding immunosuppressive therapy. Luminex-based HLA antibody testing is versatile and can be used in donor selection, pre-transplant desensitization decisions, virtual crossmatching, and post-transplant prognosis assessment.
HLA single antigen identification testing plays a vital role in identifying specific antibodies to accurately determine individual antigen specificity and detect DSA. DSA, antibodies specifically reacting to donor HLA antigens, is associated with an increased risk of rejection reactions and decreased graft survival. Continuous monitoring of DSA through post-transplant HLA single antigen identification testing is recommended to improve patient survival rates.
Additionally, DSA serves
as an important diagnostic criterion for antibody-mediated rejection in
post-transplant patients. De novo detection of donor-specific HLA antibodies is
associated with an increased risk of rejection, highlighting the importance of
ongoing surveillance through HLA single antigen identification testing. The
significance of DSA extends beyond solid organ transplantation to include
haploidentical hematopoietic stem cell transplants, with recommendations from
organizations such as the European Society for Blood and Marrow Transplantation
to include DSA in donor selection criteria. Therefore, continuous tracking of
DSA through post-transplant HLA single antigen identification testing can be a
crucial factor in enhancing patient survival.
References
01. Korean Society of Laboratory Medicine, Diagnostic Laboratory Medicine 6th Edition, Beommun Education
02. El-Awar N, Lee J, Terasaki PI. HLA antibody identification with single antigen beads compared to conventional methods. Hum Immunol. 2005;66:989-97.
03. Colombo MB, Haworth SE, Poli F, Nocco A, Puglisi G, Innocente A, et al. Luminex technology for anti-HLA antibody screening: evaluation of performance and of impact on laboratory routine. Cytometry B Clin Cytom. 2007;72:465-71.
04. Lee H, Oh E. Luminex-based Immunoassay for Organ Transplantation. Korean Journal of Transplantation. 2015;29:54-60.
05. Lachmann N, Terasaki PI, Budde K, Liefeldt L, Kahl A, Reinke P, et al. Anti-human leukocyte antigen and donor-specific antibodies detected by luminex posttransplant serve as biomarkers for chronic rejection of renal allografts. Transplantation. 2009;87:1505-1513.
06. Lefaucheur C, Loupy A, Hill GS, Andrade J, Nochy D, Antoine C, et al. Preexisting donor-specific HLA antibodies predict outcome in kidney transplantation. J Am Soc Nephrol. 2010;21:1398–1406.
Test Information
GC Labs code | Test item |
A030 | |
M741 | |
M742 | |
M743 |

