Sae Mi Lee, M.D.
Department of Laboratory Medicine
Non-invasive prenatal testing (NIPT) is a screening test that analyzes cell-free fetal DNA present in maternal blood to detect fetal chromosomal abnormalities. Over the past decade, it has been recognized as a technology that has established a new standard in prenatal screening (Fig. 1).
American College of Obstetricians and Gynecologists and American College of Medical Genetics and Genomics recommend that NIPT may be considered for all pregnant women. In particular, based on its high sensitivity and specificity for trisomy 21, 18, and 13, NIPT demonstrates clearly superior performance compared with conventional serum-based screening tests. This change in recommendations indicates that NIPT is no longer a selective test limited to high-risk pregnancies but has become a first-line screening test that can be broadly applied regardless of maternal age or clinical history.
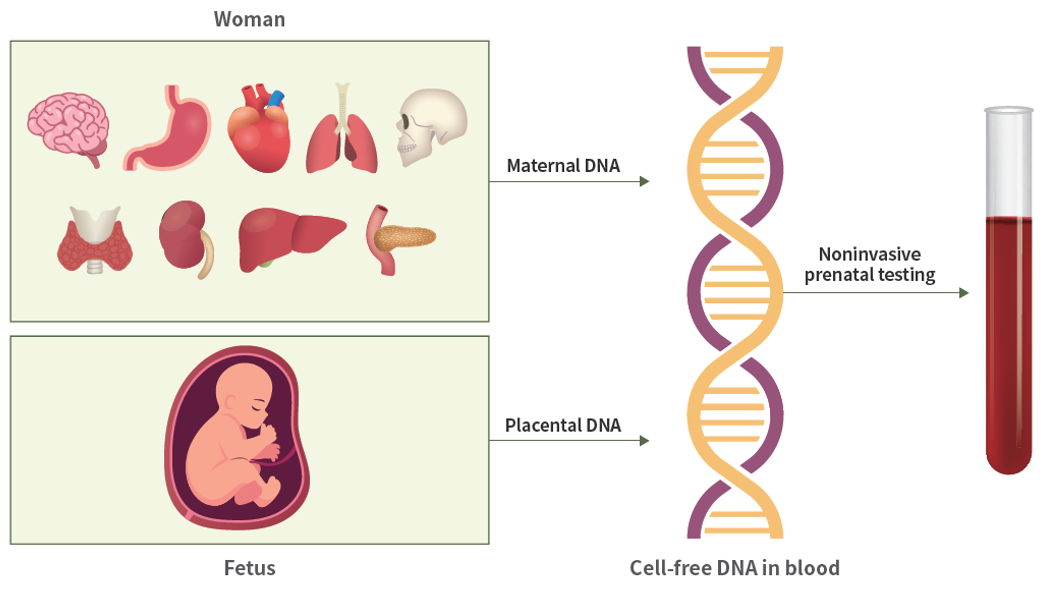
Fig. 1. Cell-free DNA in maternal blood and non-invasive prenatal testing
Trends in the Application of NIPT
1. Major Trisomies (Trisomy 21, 18, 13)
Large-scale studies have reported that the sensitivity for trisomy 21 is approximately 98~99%, with specificity exceeding 99.9%. Trisomy 18 and trisomy 13 show similarly high performance, which is markedly superior to conventional maternal serum screening tests.
2. Sex Chromosome Aneuploidy (SCA)
Sex chromosome aneuploidy refers to a group of disorders caused by loss or gain of X or Y chromosomes and most commonly includes Turner syndrome (45,X) and Klinefelter syndrome (47,XXY), 47,XXX, 47,XYY, which present with diverse clinical manifestations. Turner syndrome is associated with short stature, gonadal dysfunction, and congenital heart disease, while Klinefelter syndrome is characterized by gonadal dysfunction and impaired spermatogenesis. 47,XXX and 47,XYY generally show mild phenotypes, but in some individuals may be accompanied by developmental delay or learning disabilities.
SCA occurs in approximately 1 in 400 live births, making it a relatively common condition; however, many cases remain undiagnosed due to the absence of obvious abnormalities. Although NIPT can detect SCA, its positive predictive value (PPV) is lower than that of autosomal trisomies. The overall PPV is approximately 50%, with 45,X showing the lowest value of 14.5~32.0%, followed by 47,XXX at 57.5~61.6%, 47,XXY at 67.6~97.7%, and 47,XYY at 70.9~100%. The low PPV is due to confined placental mosaicism (CPM) and maternal undiagnosed sex chromosome aneuploidy, which contribute to false-positive results. In one study, 53% of false-positive 45,X cases were confirmed to be due to maternal mosaicism.
Because abnormal findings due to SCA is often making NIPT results difficult to interpret, and prenatal diagnosis does not always lead to direct clinical benefit, ethical considerations are required. Therefore, when a high-risk SCA result is obtained, specialized prenatal genetic counseling is required, and karyotyping by amniocentesis should be preferentially considered for confirmation.
3. Rare Autosomal Trisomies (RAT)
Rare autosomal trisomies refer to chromosomal abnormalities in which an extra copy is present in an autosome other than chromosomes 13, 18, and 21. These abnormalities occur at a frequency of approximately 0.12% to 0.95%, depending on the selected population. The positive predictive value of RAT detection using NIPT has been reported as approximately 9% (95% confidence interval: 2.5%~18.8%), but due to low prevalence and the analytical approach that aggregates all RATs, reliable data to accurately determine sensitivity and specificity are still lacking.
Non-mosaic rare autosomal trisomies (complete RAT) are mostly lethal, making fetal survival difficult and resulting in spontaneous miscarriage in most cases; when pregnancy continues, the condition is usually mosaic, and in the majority of cases represents confined placental mosaicism, with the fetus having a normal karyotype. Most RAT detected by NIPT involve chromosomes 2, 3, 7, 8, 9, 14, 15, 16, 20, and 22, with trisomy 7 being the most frequently detected (Fig. 2).
When fetal mosaicism (RAT) is present, it may be associated with placental insufficiency, low birth weight, miscarriage, and structural anomalies, although the degree of mosaicism does not necessarily correlate with the severity of the fetal phenotype, which makes prognostic counseling difficult. Even in cases of confined placental mosaicism where the fetus has a normal karyotype, this condition is being studied as a pregnancy-related prognostic indicator, and has been reported to be associated with an increased risk of intrauterine growth restriction (IUGR) and preeclampsia.
When a high-risk RAT result is obtained by NIPT and no fetal structural abnormalities are observed on ultrasound, more than 97% of detected cases are due to CPM, therefore amniocentesis is preferred over chorionic villus sampling for confirmatory testing. Meiotic CPM may be accompanied by uniparental disomy (UPD), and when UPD is associated with chromosomes 6, 7, 11, 14, 15, or 20, which contain imprinted regions, it is therefore associated with clinically important syndromes. Therefore, in cases of high-risk trisomy of chromosomes 6, 7, 11, 14, 15, or 20, chromosomal microarray analysis or methylation testing should be considered during invasive testing in order to evaluate the presence of UPD.
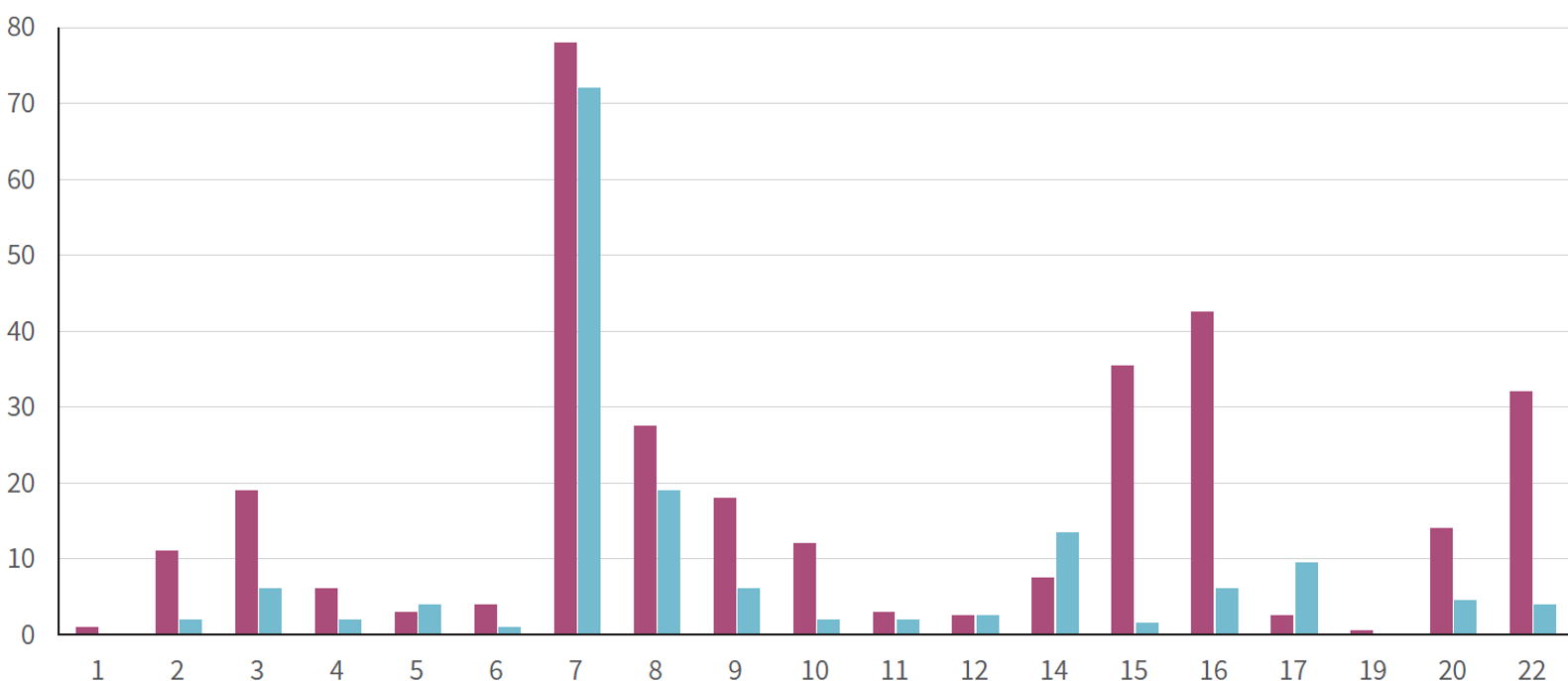
Ref) Rare autosomal trisomies detected by non-invasive prenatal testing: an overview of current knowledge
Fig. 2. Frequency of RATs detected by NIPT.
4. Copy number variant (CNV)
Copy number variants (CNV) are genomic imbalances caused by deletions or duplications, including microdeletions/duplications smaller than 5~10 Mb that cannot be detected by conventional karyotyping. While most individuals carry multiple CNV, some CNV are associated with congenital anomalies, intellectual disability, autism spectrum disorder, and neurodevelopmental disorders, and therefore of substantial clinical significance. In prenatal settings, CNV are found in approximately 6% of pregnancies with structural abnormalities and 1.7% of pregnancies without structural abnormalities. Individual pathogenic CNV are rare, unrelated to maternal age, and often occur de novo in the fetus. In addition, microdeletions and duplications can be affected by confined placental mosaicism (CPM), resulting in false-positive results, and NIPT for a given CNV is not feasible when the mother is a carrier of that CNV. Although CNV are clinically important, the individual frequencies of specific CNV are very low, and large-scale confirmatory studies for performance evaluation are difficult to conduct; therefore, the evidence for test accuracy and clinical utility is limited.
CNV screening test using NIPT is primarily focused on the 22q11.2 microdeletion. The 22q11.2 microdeletion syndrome is the most common recurrent microdeletion syndrome, occurring in approximately 1 in 3,000 to 1 in 6,000 live births. This condition shows wide phenotypic variability and can cause a broad range of clinical manifestations, including congenital heart defects, renal and craniofacial anomalies, immunodeficiency, endocrine disorders, hearing loss, intellectual disability, developmental delay, and psychiatric disorders (Fig. 3).
In a prospective study published in 2022, the performance of cfDNA-based 22q11.2 deletion screening was evaluated, with a reported sensitivity of 83.3%, specificity of 99.9%, positive predictive value of 52.6%, and negative predictive value of 99.9%. In addition, a recent meta-analysis evaluated the performance of NIPT not only for 22q11.2 microdeletion but also for other recurrent microdeletion syndromes, including 15q deletion and 5p (cri-du-chat) deletion. In this study, the positive predictive value was 49% for 22q11.2 deletion, 25.9% for 15q deletion, and 30.8% for 5p deletion, and it was emphasized that a substantial proportion of the pregnancies included in the analysis belonged to a high-risk group with ultrasound abnormalities. When all CNV were analyzed together, the positive predictive value was 51.5% in high-risk populations and 36.0% in low-risk populations. Even in large-scale clinical studies, the positive predictive value for structural chromosomal abnormalities remains limited. In the Netherlands TRIDENT study, 188 cases of structural chromosomal abnormalities (deletions/duplications) were detected by NIPT, and follow-up confirmatory testing demonstrated a positive predictive value of 44.1%.
In summary, although NIPT has relatively high
sensitivity and specificity, its PPV is low, and result interpretation is
complex due to the rarity of individual CNV as well as the effects of CPM and
maternal-origin CNV. Therefore, when offering expanded NIPT, clinicians should
provide comprehensive counseling to patients, including the limitations of the
test, the possibility of a low PPV, and the necessity of confirmatory testing
by invasive procedures in the case of positive results. In addition, high-risk
results must be confirmed by invasive diagnostic testing, such as amniocentesis
and chromosomal microarray analysis, and professional prenatal genetic
counseling is essential.
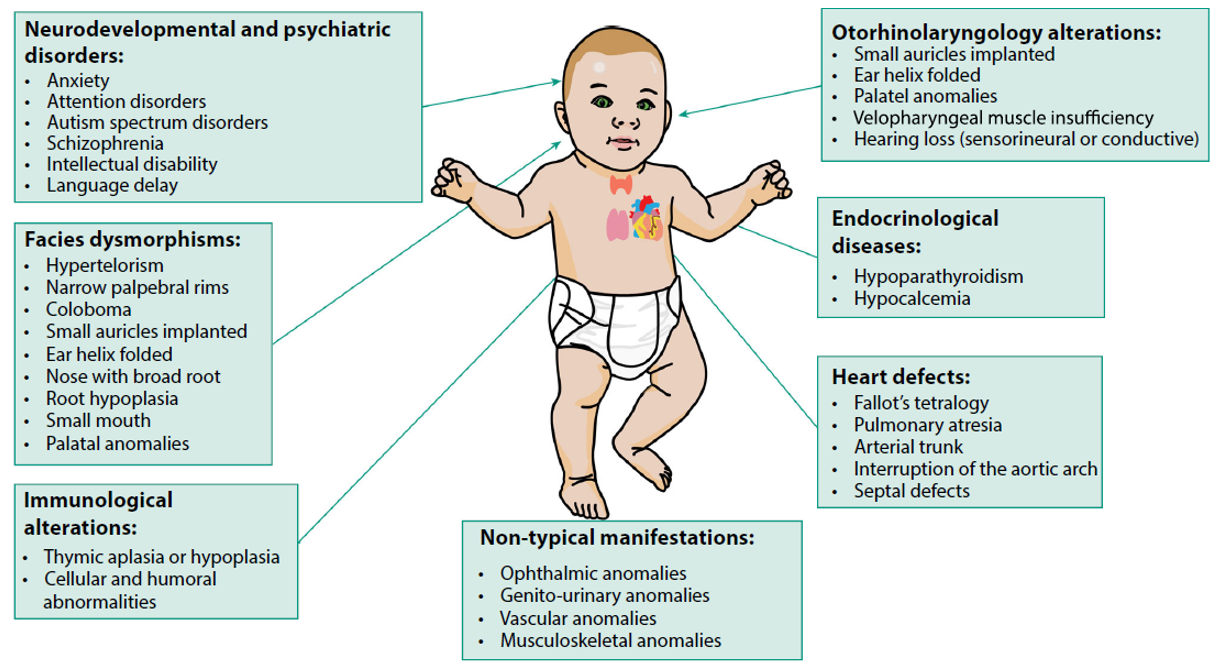
Ref) Applications for cell-free fetal DNA testing beyond common aneuploidy
Fig. 3. Clinical Features of 22q11.2 microdeletion Syndrome
Role of Genetic Counseling
Interpretation of NIPT results and genetic counseling are becoming increasingly complex with the expansion of the technology. It must be clearly explained to patients that NIPT is fundamentally a screening test, not a diagnostic test, and that a negative result does not exclude the possibility of structural anomalies, developmental disorders, or other genetic conditions.
When interpreting positive results, the difference in PPV by chromosome must be considered. In particular, RAT and CNV results are much more likely to reflect placental-origin abnormalities rather than true fetal abnormalities, and therefore the need for invasive confirmatory testing should be discussed carefully. In addition, even when the fetal karyotype is confirmed to be normal in cases of RAT, the risks of IUGR, preterm birth, and obstetric complications may be increased, so establishing a detailed follow-up plan for the remainder of the pregnancy is essential.
In the genetic counseling process, an approach that considers not only the technical limitations of the test but also the emotional and psychological state of the patient is required. Although the expansion of NIPT provides more information to pregnant women, it can also lead to misunderstanding and unnecessary anxiety. Therefore, it is important to provide a professional and clear explanation of the testing process, the meaning of the results, follow-up options, and the advantages and disadvantages of different testing choices, in order to help pregnant women, make informed decisions based on sufficient information.
References
01. Ferguson G, Kilby MD, Mone F. Applications for cell-free fetal DNA testing beyond common
aneuploidy. The Obstetrician & Gynaecologist, 2025;27:197-206.
02. Swanson K, and Norton ME. Expanded applications of cell free fetal DNA screening. Best Pract Res Clin
Obstet Gynaecol. 2025;98:102574.
03. Van Prooyen Schuurman L, Sistermans EA, Van Opstal D, Henneman L, Bekker MN, Bax CJ, et al. Clinical
impact of additional findings detected by genome-wide non-invasive prenatal testing: Follow-up results of the TRIDENT-2 study. Am J Hum Genet. 2022;109(6):1140-1152.
04. Lannoo L, Van Straaten K, Breckpot J, Brison N, De Catte L, Dimitriadou E, et al. Rare autosomal trisomies
detected by non-invasive prenatal testing: an overview of current knowledge. Eur J
Hum Genet. 2022;30(12):1323-1330.
05. Poulton A and Hui L. Noninvasive prenatal testing: an overview. Aust Prescr. 2025;48:47-53.
Test Information
GC Labs code | Test item |
Q985 | |
Q984 | |
Q986 |

